File:10. Оксидација на јагленче во стопен калиум нитрат.webm
From Wikimedia Commons, the free media repository
Jump to navigation
Jump to search
Size of this JPG preview of this WEBM file: 800 × 450 pixels. Other resolutions: 320 × 180 pixels | 640 × 360 pixels | 1,024 × 576 pixels | 1,280 × 720 pixels | 1,920 × 1,080 pixels.
Original file (WebM audio/video file, VP9/Vorbis, length 1 min 18 s, 1,920 × 1,080 pixels, 4.5 Mbps overall)
File information
Structured data
Captions
Captions
Add a one-line explanation of what this file represents
Summary[edit]
| Description10. Оксидација на јагленче во стопен калиум нитрат.webm |
Македонски: Експеримент кој ја демонстрира оксидацијата на јагленче во стопен калиум нитрат. При контакт на зажарено јагленче со стопен калиум нитрат започнува интензивно ослободување на кислород. Ослободениот кислород доведува до оксидација при која се зголемува температурата на јагленчето и тоа продолжува да гори во атмосфера од речиси чист кислород. Ова е проследено со постојано потскокнување на зажареното јагленче над стопениот калиум нитрат. Процесот може да се опише со упростена (и сумарна) равенка која опишува дел од реакциите кои се одвиваат во системот:
English: An experiment that demonstrates the oxidation of a piece of charcoal in molten potassium nitrate. When glowing charcoal is placed in molten potassium nitrate, oxygen formation starts. The oxygen is responsible for the oxidation that increases the temperature of the charcoal and the charcoal continues to burn in almost pure oxygen atmosphere. This is accompanied by continuous bouncing of the charcoal above the molten potassium nitrate. The process can be described by the simplified (and summarised) equation that describes part of the reactions that take place:
English: Experiment ce demonstrează oxidarea unei bucăți de cărbune în azotat de potasiu topit. Când cărbunele incandescent este plasat în azotatul de potasiu topit, începe formarea de oxigen. Oxigenul este responsabil de oxidarea care crește temperatura cărbunelui, care continuă să ardă într-o atmosferă formată aproape exclusiv din oxigen pur. Aceasta este însoțită de salturi continue ale cărbunelui peste azotatul de potasiu topit. Procesul poate fi descris prin ecuația simplificată (și sumarizată) care descrie o parte a reacțiilor ce au loc:
|
| Date | |
| Source | Own work |
| Author | Petrovskyz |
| Other versions |
Derivative works of this file: Smoking test tube looped animation.gif: 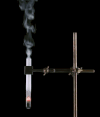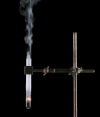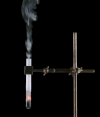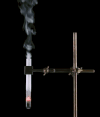 |
Licensing[edit]
I, the copyright holder of this work, hereby publish it under the following license:
This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
- You are free:
- to share – to copy, distribute and transmit the work
- to remix – to adapt the work
- Under the following conditions:
- attribution – You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- share alike – If you remix, transform, or build upon the material, you must distribute your contributions under the same or compatible license as the original.
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 00:47, 18 October 2016 | 1 min 18 s, 1,920 × 1,080 (41.64 MB) | Petrovskyz (talk | contribs) | User created page with UploadWizard |
You cannot overwrite this file.
File usage on Commons
The following 4 pages use this file:
File usage on other wikis
The following other wikis use this file:
- Usage on el.wikipedia.org
- Usage on en.wikipedia.org
- Usage on eo.wikipedia.org
- Usage on et.wikipedia.org
- Usage on hu.wikipedia.org
- Usage on hy.wikipedia.org
- Usage on meta.wikimedia.org
- Usage on mk.wikipedia.org
- Usage on ml.wikipedia.org
- Usage on ro.wikipedia.org
- Usage on sk.wikipedia.org
- Usage on tr.wikipedia.org
- Usage on uk.wikipedia.org
Transcode status
Update transcode status| Format | Bitrate | Download | Status | Encode time |
|---|---|---|---|---|
| VP9 1080P | 964 kbps | Download file | Completed 12:40, 3 August 2018 | 3 min 14 s |
| VP9 720P | 481 kbps | Download file | Completed 12:38, 3 August 2018 | 2 min 2 s |
| VP9 480P | 303 kbps | Download file | Completed 12:37, 3 August 2018 | 1 min 31 s |
| VP9 360P | 221 kbps | Download file | Completed 12:36, 3 August 2018 | 1 min 2 s |
| VP9 240P | 170 kbps | Download file | Completed 12:36, 3 August 2018 | 48 s |
| VP9 180P | 149 kbps | Download file | Completed 12:36, 3 August 2018 | 40 s |
| VP9 120P | 124 kbps | Download file | Completed 12:35, 3 August 2018 | 29 s |
| WebM 1080P | 4.18 Mbps | Download file | Completed 09:24, 16 March 2017 | 4 min 25 s |
| WebM 720P | 2.14 Mbps | Download file | Completed 09:16, 16 March 2017 | 2 min 11 s |
| WebM 480P | 1.09 Mbps | Download file | Completed 09:09, 16 March 2017 | 2 min 38 s |
| WebM 360P | 580 kbps | Download file | Completed 09:08, 16 March 2017 | 1 min 24 s |
| WebM 240P | 330 kbps | Download file | Completed 16:22, 16 February 2017 | 58 s |
| WebM 160P | 218 kbps | Download file | Completed 17:02, 16 February 2017 | 1 min 0 s |
Metadata
This file contains additional information such as Exif metadata which may have been added by the digital camera, scanner, or software program used to create or digitize it. If the file has been modified from its original state, some details such as the timestamp may not fully reflect those of the original file. The timestamp is only as accurate as the clock in the camera, and it may be completely wrong.